The US federal government will pay for dry ice to help out jurisdictions that do not have the freezers needed to store Pfizer’s coronavirus vaccine, a source familiar with Operation Warp Speed told CNN Tuesday.
The Food and Drug Administration has asked its vaccine advisers to meet Dec. 10 to discuss Pfizer’s application for emergency use authorization for its vaccine.
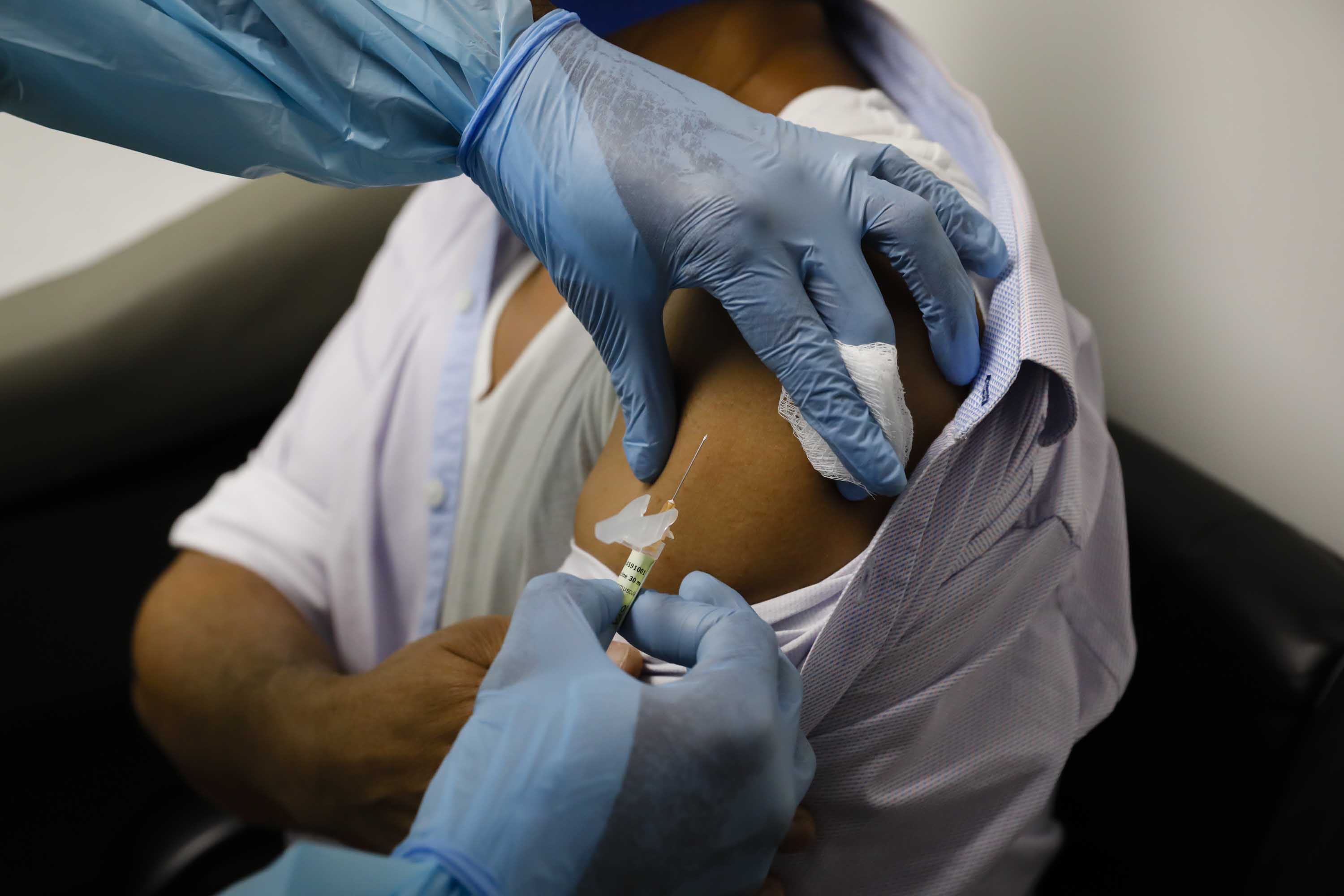
The temperature problem: States, cities and territories have worried about how they could handle the delicate vaccine if it is approved and distributed.
The vaccine must be shipped and stored at temperatures of around minus 100 degrees Fahrenheit, or minus 75 degrees Celsius. That requires a special freezer that most hospitals, pharmacies or clinics do not have.
The solution: The federal government's plans to handle this problem were shared in a call with the health care industry, the source said.
Jurisdictions without an ultra-low freezer will automatically receive a complimentary shipment of dry ice within 24 hours of receiving the vaccine suitcase. The dry ice will be paid for and shipped by the federal government, the source told CNN.
The shipment will include the scoop, goggles, and cryogenic gloves needed to safely handle the dry ice.
The government is also contracting with the company making temperature monitoring devices that will be fitted onto each suitcase to ensure the vaccines inside never thaw out and get ruined. The suitcase will carry a log to notate temperature.
Each box can be used with the dry ice for five days and refreshed twice, meaning that the vaccine must be administered within a 15-day period. There may also be an option to refrigerate the vaccine for 20 days.