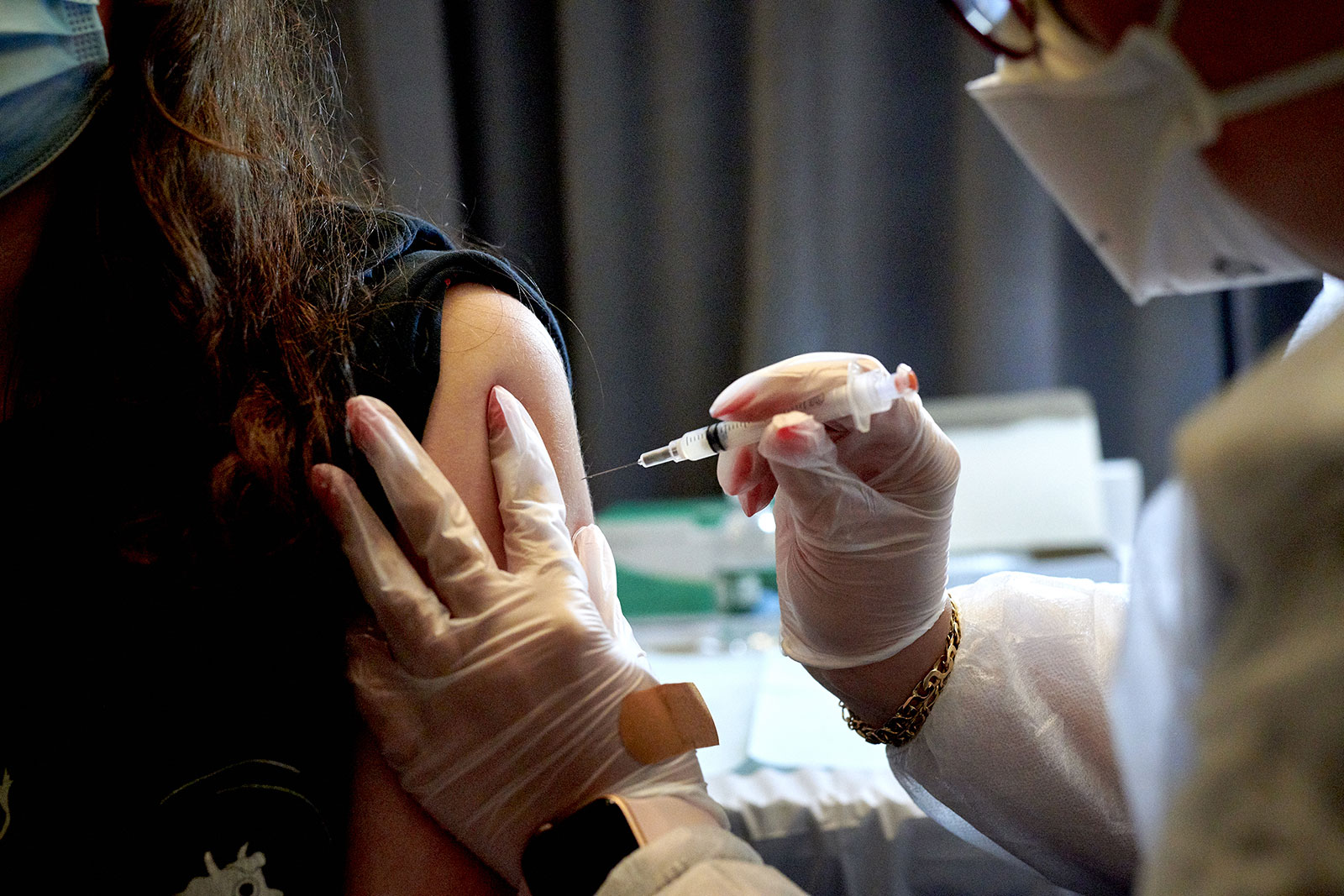
Moderna announced it has begun applying for full approval for its Covid-19 vaccine in people ages 18 and up.
The company says it will continue to submit trial data “on a rolling basis over the coming weeks with a request for a Priority Review.” A priority review asks the US Food and Drug Administration to take action within six months, compared to the 10 months designated under standard review.
“We are pleased to announce this important step in the U.S. regulatory process for a Biologics License Application (BLA) of our COVID-19 vaccine,” Moderna CEO Stéphane Bancel said in a statement Tuesday. “We look forward to working with the FDA and will continue to submit data from our Phase 3 study and complete the rolling submission.”
Since December, Moderna’s two-shot vaccine has been distributed under an emergency use authorization for people ages 18 and up. In April, the company announced its vaccine maintained over 90% efficacy six months out – the amount of follow-up time needed to apply for FDA approval.
Moderna is the second company to seek such approval in the US. Last month, Pfizer announced it was initiating its own application for people ages 16 and up, following an April announcement that its clinical trials showed over 91% efficacy after six months. Experts say they expect this protection will last much longer, to be confirmed as more data come in.
Being granted FDA approval may motivate some vaccine-hesitant people to roll up their sleeves, according to research released Friday by the Kaiser Family Foundation.
Both Pfizer and Moderna are also studying their vaccines in children as young as 6 months. Last month, the FDA granted Pfizer’s vaccine an emergency use authorization for children 12 to 15.