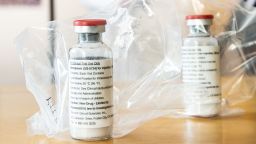
The United States has been allocated almost all of the next few months’ supply of remdesivir, the only drug that’s known to work directly against Covid-19.
The Department of Health and Human Services (HHS) announced Monday that it had secured 100% of drug-maker Gilead’s projected production for July, and 90% of its production for August and September – plus more for clinical trials.
“President Trump has struck an amazing deal to ensure Americans have access to the first authorized therapeutic for Covid-19,” said HHS Secretary Alex Azar in a news release.
HHS has secured more than 500,000 treatment courses of the antiviral drug for US hospitals through September, according to the release. Remdesivir is the only drug that has an emergency use authorization from the US Food and Drug Administration to treat coronavirus, and it is patented by Gilead Sciences.
Another drug, the widely available steroid dexamethasone, is useful for providing supportive care to the sickest Covid-19 patients who require ventilation or oxygen, according to preliminary research from the UK.
Gilead had donated a supply of 1.5 million doses of remdesivir to countries across the world, which it says is enough for around 140,000 treatment courses. Almost a million doses were reserved for the US, according to the HHS, but the supply is now running out.
The US government’s last shipment of the donated antiviral drug went out this week. Gilead is ramping up to make more, but it’s unclear how much will be available this summer.
The company has said it plans to have more than 500,000 treatment courses available by October, and more than 2 million by December, but it is unclear how these will be distributed internationally.
CNN has approached Gilead for comment.
The World Health Organization (WHO) said Wednesday it was working to verify reports that the US is hoarding remdesivir.
“We’re aware of the reports in the media around this purchase or procurement of remdesivir stocks, and we’re obviously working through our colleagues and our partners and the access to Covid Tools Accelerator to clarify and verify this this report,” Dr. Mike Ryan, WHO executive director of Health Emergencies Program, told a briefing.
“Obviously, there are many people around the world who are very sick with this disease and we want to ensure that everybody has access to the necessary lifesaving interventions,” Ryan said.
“We are fully committed as an organization, and with our partners to equitable access to life saving interventions,” he added.
Ryan said WHO continues to work with the US – even though President Trump has said he has split with the organization. “We are grateful for and continue to engage with our technical counterparts in the United States on all matters related to science and public health,” he said.
Gilead announced in an open letter on Monday morning that it had decided to set a price of $390 per vial for governments of developed countries. A typical five-day treatment course would include six vials, which would equate to $2,340 per patient, Daniel O’Day, Gilead Sciences chairman and CEO, said in the letter.
The price for the typical US patient (those on private insurance or the government Medicare and Medicaid programs) will be $520 per vial. That’s a total of $3,120 for a five-day treatment.
The drug manufacturer has also reached agreements with five generic drugmakers in India, Egypt and Pakistan, allowing them to produce remdesivir for a list of 127 mostly low-income and lower-middle income countries.
“In the developing world, where healthcare resources, infrastructure and economics are so different, we have entered into agreements with generic manufacturers to deliver treatment at a substantially lower cost. These alternative solutions are designed to ensure that all countries in the world can provide access to treatment,” O’Day wrote.
However, many countries are not on that list, including Brazil, China, Japan, Mexico and many European nations.
And remdesivir has undergone trials in many of these countries, including China, France, Germany, Japan and the UK. One study has shown it can shave four days off a hospital stay, from 15 to 11 days.
Andrew Hill, a senior visiting Research Fellow at Liverpool University’s Pharmacology Department, told CNN that his understanding was that the US had bought most of Gilead’s supply of the drug, which is in demand from high-income countries in Europe and the Americas.
“Never in my medical career, I’ve never known a situation where a single country then requisitions the whole supply of a drug for life threatening disease, it’s just unprecedented,” he added.
“You think about the clinical trials of remdesivir, and patients in the UK participated in those studies and risked their own health for a drug, which at the time didn’t have any proven benefits. They took the risks, and yet, given that all these patients in different countries have taken this drug, why should it be only the Americans that get to benefit from the research?
“It actually calls into question the most basic of ethical standards to actually take all the drug back to the United States when it’s been tested in a whole variety of countries.
“It’s not the way that you deal with a pandemic. If everybody goes into their silo and starts hoarding drugs and equipment and vaccines, then the drugs are not going to be given out fairly.”
UK Business Minister Nadhim Zahawi responded to the news on remdesivir by telling Sky News that governments and companies should “cooperate” on finding treatments for coronavirus “because the best outcome for the whole world is that we work together.”
He pointed to UK pharmaceutical company AstraZeneca’s international deals to supply hundreds of millions of doses of a vaccine globally if one is produced at Oxford University.
“By attempting to compete, I think we ultimately undermine all of our strategies. It is much better to work together than to work to undermine each other, so we will continue in that spirit,” said Zahawi.
Penny Ward, Visiting Professor in Pharmaceutical Medicine at King’s College London and Chair of the Education and Standards Committee of the Faculty of Pharmaceutical Medicine, said in a statement to the UK-based Science Media Centre: “Gilead has been clear that manufacturing capacity is limited by a number of issues and have laid out the steps they have been taking to scale up to try to meet demand.”
Ward added it should be borne in mind that the US is the country worst affected by Covid-19, with more than 2.6 million cases and 127,000 deaths to date, according to the Johns Hopkins University tally.
“It is unreasonable to expect that the US government should deny their population access to drugs manufactured in the USA,” she said in the statement.
“These laws also exist in many other countries, and indeed the UK government has enacted laws to prohibit the export of essential medicines from the UK in this same situation.”
Ward said statements from Gilead “indicate that they have worked hard to try to scale up a difficult manufacturing process as rapidly as they can” and make supplies available for clinical trials and compassionate use as well as in developing countries.
“It would be unfair to the company to blame Gilead for President Trump’s actions,” she added in the statement.
Dr. Ohid Yaqub, Senior Lecturer at the Science Policy Research Unit, University of Sussex, said in a statement to the Science Media Centre: “The buying-up of remdesivir is disappointing news, not necessarily because of the shortages it implies for other countries, but because it so clearly signals an unwillingness to co-operate with other countries, and the chilling effect this has on international agreements about intellectual property rights.”
The latest announcement raises questions over whether the US could be prioritized over other countries when it comes to the supply of other vital drugs in the fight against coronavirus.
Paul Hudson, the CEO of French pharmaceutical giant Sanofi, caused controversy in mid-May when he appeared to suggest the US would be first in line to receive a coronavirus vaccine if the company succeeds in developing one.
He was subsequently summoned to the Elysee Palace by French President Emmanuel Macron, who stressed the need to manufacture a vaccine available to everyone as soon as possible. Sanofi later said Hudson’s comments were “misinterpreted.”
Canadian Prime Justin Trudeau has also said that if a vaccine is found “it cannot be just the wealthiest countries producing that vaccine for its citizens.”
CNN’s Amanda Watts, Jacqueline Howard, Elizabeth Cohen and Arman Azad contributed reporting.