Editor’s Note: Dr. Kellyann Niotis is a Cornell-trained preventive neurologist specializing in Parkinson’s disease. Her research at the Institute for Neurodegenerative Diseases focuses on personalized risk reduction for patients at risk for neurodegenerative diseases. Dr. Richard S. Isaacson is a Harvard-trained neurologist and researcher in Alzheimer’s prevention at the Institute for Neurodegenerative Diseases. He founded and directed the first US Alzheimer’s Prevention Clinic at Weill Cornell Medicine/NewYork-Presbyterian. The views expressed in this commentary belong to the authors. View more opinion at CNN.
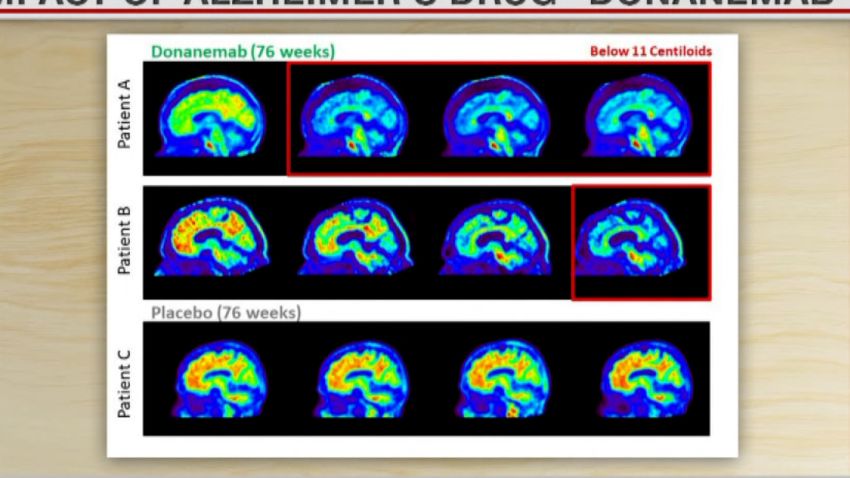
A new clinical trial of the experimental Alzheimer’s drug donanemab has produced promising results, with drugmaker Eli Lilly announcing last week that its phase 3 study showed the medication slowed cognitive decline by an impressive 35% compared with the placebo. While this development is encouraging, Alzheimer’s remains an extraordinarily complicated condition, making it highly unlikely there’s one cure-all to this health crisis.

We also must look to prevention. Despite being a buzzword in many different fields of medicine, prevention remains a dirty word within neurology. Some experts are even worried about the possibility of “overselling the idea to patients,” according to Neurology Today.

But dementia and other neurodegenerative diseases desperately need their own proactive approach. A major study found that changing behaviors in areas like exercise, socialization and blood pressure control could reduce dementia cases by as much as 40%. Yet doctors, patients and insurers are not embracing – or even aware of – this potential.
The case for dementia prevention got a boost last month when hearing aids were found to be a minimally invasive, cost-effective strategy that may prevent up to 8% of dementia cases. This backs up earlier research findings that those with hearing loss up to are up to five times more likely to develop dementia and that the use of hearing aids may cut that risk in half.
This presents a tantalizing opportunity, but so far we are largely failing to take advantage of it. We must change the way we think about neurodegenerative diseases. By 2050, 152 million people around the world could be living with Alzheimer’s or another form of dementia.
Patients often fear these conditions more than any other, as the prognosis can seem too grim to bear. For actor Chris Hemsworth, performing as a superhero was less formidable than the recent discovery that he’s part of the 2 to 3% of the population with two copies of the APOE4 gene that significantly increases his Alzheimer’s risk. He described the realization as facing his “biggest fear.”
Up to 25% of the population carries one copy of the APOE4 gene. However, despite wide accessibility to APOE testing (e.g., through companies like 23andMe), many medical experts discourage testing because they have the attitude that nothing can be done with the information.
The truth is, much can be done about it, starting with more precisely tailoring the new anti-amyloid treatments like Eli Lilly’s, as evidence suggests APOE4 carriers may be more susceptible to serious side effects of the kind that may have contributed to the deaths reported by Lilly in the trial. But time is of the essence. Alzheimer’s prevention needs to start when (or ideally before) the disease does – in many cases in one’s 30s but sometimes even earlier.
New ways to identify Alzheimer’s and other neurodegenerative diseases like Parkinson’s in their earliest stages – eye tests, blood draws and voice analysis – can offer big rewards when combined with low-risk interventions. Take the recent findings on hearing loss, which researchers believe is associated with dementia because it contributes to social isolation, loneliness and depression – key modifiable risk factors for dementia – while sound itself is believed to stimulate and support healthy brain cells. We may be able to protect our brains as well as our ears by avoiding loud noises and using hearing protection throughout life.
Hearing was among the several modifiable risk factors for dementia identified in 2020 by a leading medical journal, The Lancet. Others included: low education levels, high blood pressure, smoking, obesity, physical inactivity, diabetes, excessive alcohol consumption, brain injury and air pollution.
We believe that several other factors that could have a positive impact will likely soon be added to the Lancet’s list: Consuming a Mediterranean diet, optimizing sleep, reducing stress, combating elevated blood levels of homocysteine with B-vitamins, managing high cholesterol and, for the right people (e.g., those with the APOE4 gene) and at the right dose, supplementing with Omega-3 fatty acids.
Each of these behaviors may defend against dementia in distinctive ways. Studies suggest that getting sufficient sleep reduces the amount of toxic beta-amyloid protein – the substance that forms Alzheimer’s plaques in the brain. Meanwhile, the hippocampus, the memory region of the brain, is particularly vulnerable to the damaging effects of chronically high stress hormones.
And, with inflammation a central characteristic of Alzheimer’s and other neurodegenerative diseases, Omega-3s and extra virgin olive oil in a Mediterranean diet can have an anti-inflammatory effect.
It’s important that preventive neurological care for the above risk factors be personalized and comprehensive. Results we published in 2019 suggest that adherence to individually tailored interventions, from improved diet and exercise to optimized blood pressure and blood sugar regulation, may improve cognition and reduce risk for Alzheimer’s (and cardiovascular disease). But based on our study design, it was most likely that each intervention moved the needle differently in different people.
One of the knocks on our study and the others used to determine these risk factors is that they are not randomized controlled trials, the gold standard in medicine when it comes to proving the benefit of a given intervention. But for diseases that develop over a lifetime, randomized controlled trials can also fall short, especially on an individual level. Because neurodegenerative diseases are caused by multiple external and genetic risk factors that often interact uniquely over a person’s lifetime, we need to interpret all evidence on an individual level, rather than a population-wide level like randomized controlled trials do, and personalize our treatment approach.
This reluctance to rely on alternative study designs like ours is part of why the uptake of preventive neurology has been lagging, even among neurologists. But all the risk factors for dementia defined by the Lancet Commission in 2020 have enough evidence for us to be confident that each is causally linked to the condition and that there is a benefit to intervention. This method is what allowed scientists to determine that smoking causes lung cancer, for instance, in place of a randomized trial.
As two of only a handful of neurologists who focus on prevention, our five-plus-year waitlist demonstrates how massively inadequate the supply of doctors is for meeting the demand. So people at risk must empower themselves to ask their doctors about preventive measures they can take. And anyone can be proactive by signing up to join an online brain health study or a clinical trial like the AHEAD Study.
Get our free weekly newsletter
Tragically, the systemic forces impeding widespread access to preventive neurological care are so great that it’s currently impossible to make it the standard in clinical practice. From increasing training and educational efforts for medical providers and patients to insurers covering preventive neurological care, major structural changes must occur before everyone at risk of developing a neurodegenerative condition is helped.
Getting there depends on our ability to change the way we look at Alzheimer’s and other neurodegenerative diseases. What if we viewed the inherent complexity of these conditions as not just an obstacle, but as an opportunity to take a proactive approach?