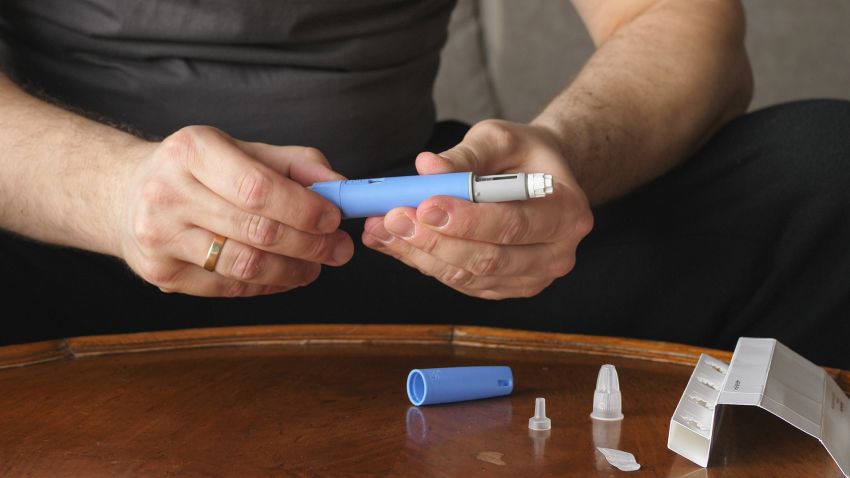
The US Food and Drug Administration issued a warning Tuesday about compounded versions of the drug semaglutide, which is approved for treatment of diabetes and excess weight.
Compounding “is the process of combining, mixing, or altering ingredients to create a medication tailored to the needs of an individual patient,” the FDA says. “Compounding includes the combining of two or more drugs.”
Semaglutide, a type of drug called a GLP-1 agonist, is FDA-approved as Ozempic and Rybelsus to treat type 2 diabetes and as Wegovy to treat overweight and obesity.
Ozempic and Wegovy have been on the FDA’s Drug Shortages list since last year.
“When a drug is in shortage, compounders may be able to prepare a compounded version of that drug if they meet certain requirements of the Federal Food, Drug, and Cosmetic (FD&C) Act,” the FDA says, but the agency doesn’t review these compounded versions for safety, effectiveness or quality.
The FDA says that it has received reports of adverse events in people who used compounded semaglutide and that patients “should not use a compounded drug if an approved drug is available.”
Some compounders may also be using salt forms of the medication, such as semaglutide sodium and semaglutide acetate, which have different active ingredients from those in the approved drugs. “The agency is not aware of any basis for compounding using the salt forms that would meet the FD&C requirements. … Products containing these salts, such as semaglutide sodium and semaglutide acetate, have not been shown to be safe and effective.”
Get CNN Health's weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
Patients and health care providers should be aware of these differences in compounded medications, the FDA says.
“Patients should only obtain drugs containing semaglutide with a prescription from a licensed health care provider, and only obtain medicines from state-licensed pharmacies or outsourcing facilities registered with FDA.”
The Alliance for Pharmacy Compounding issued a statement on semaglutide last week in which it advised consumers not to shop online for anything purported to be semaglutide if they don’t have a prescription and can’t verify that the seller is a licensed US pharmacy.
Anyone who is prescribed compounded semaglutide and wants to be certain about the ingredients can ask the pharmacist for a certificate of analysis and results from analytical testing labs, the group says.